Passivation of Stainless Steel Parts
Passivation of Stainless Steel Parts
Passivation is a vital process in various industries, including aerospace, defense, electronics, and medical. It involves creating a protective oxide layer on the surface of stainless steel to improve its resistance to corrosion and extend its service life. Understanding the science behind this process, exploring different techniques, and examining its applications can help highlight its importance in maintaining the quality and durability of stainless steel components. In this blog, we’ll cover key terms, chemical reactions, benefits, challenges, and safety measures related to passivation.
Understanding Passivation
Passivation is a chemical treatment designed to enhance the corrosion resistance of stainless steel by removing free iron from the surface and forming a thin, protective oxide layer. Free iron particles, if left untreated, can react with environmental elements, leading to rust, oxidation, and discoloration. The passivation process eliminates these contaminants and promotes the formation of a passive film that acts as a barrier against corrosion.
Key Terms in Passivation
Free Iron: This refers to unbound iron on the surface of stainless steel. If not removed, it can act as a catalyst for corrosion. Removing it helps create a clean, uniform surface.
Passive Film: A thin layer of metal oxide that forms during passivation. It serves as a protective shield, preventing further oxidation and enhancing corrosion resistance.
Surface Contamination: Dirt, oil, grease, or other foreign substances on the surface can interfere with passivation. Proper cleaning before the process is essential for effectiveness.
Conversion Coating: A general term for chemical treatments that improve surface properties. Passivation is a type of conversion coating specifically used for stainless steel to promote oxide layer formation.
The Science Behind Passivation
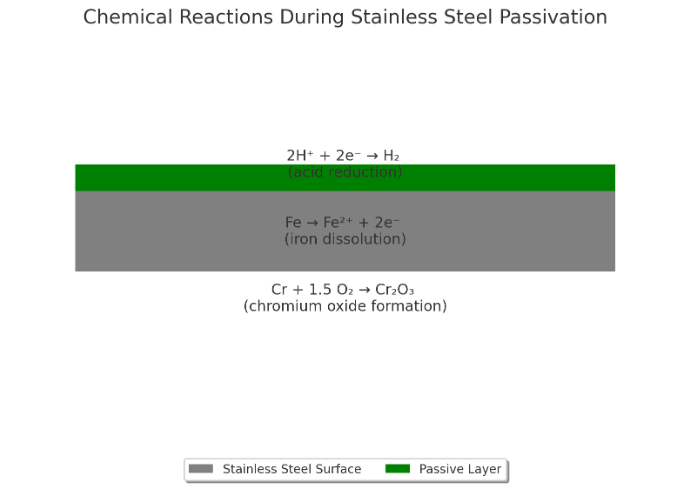
The science of passivation lies in the chemical interactions between stainless steel and the passivating solution. When parts are immersed in an acid solution like citric or nitric acid, a series of reactions occur on the surface. These reactions remove impurities and encourage the formation of a stable chromium oxide layer.
This oxide layer acts as a protective barrier, reducing the likelihood of corrosion. Understanding these chemical processes is key to optimizing the passivation treatment and ensuring long-term performance of stainless steel components.
Types and Techniques of Passivation
There are several methods of passivation, each with unique advantages. Two of the most common are citric acid and nitric acid passivation. The choice depends on the application, industry standards, and environmental considerations.
Citric Acid Passivation
Citric acid passivation is becoming increasingly popular, especially in the food and medical industries, due to its lower toxicity and environmental friendliness. Unlike nitric acid, citric acid is easier to handle and dispose of, making it a safer option. It also provides excellent corrosion resistance without leaving harmful residues, making it ideal for high-purity applications.
Nitric Acid Passivation
Nitric acid passivation is known for its strong oxidizing properties, which allow it to effectively remove iron and other contaminants from stainless steel surfaces. This method is often used in industrial settings where maximum corrosion resistance is required. However, it requires careful handling due to its higher reactivity and potential hazards.
The Role of Oxidation in the Passivation Layer
Oxidation plays a crucial role in forming the passive layer. Chromium in stainless steel reacts with oxygen in the passivating solution, forming a thin layer of chromium oxide. This layer acts as a barrier, protecting the metal from corrosive agents and extending its lifespan.
The Procedure of Passivating Stainless Steel
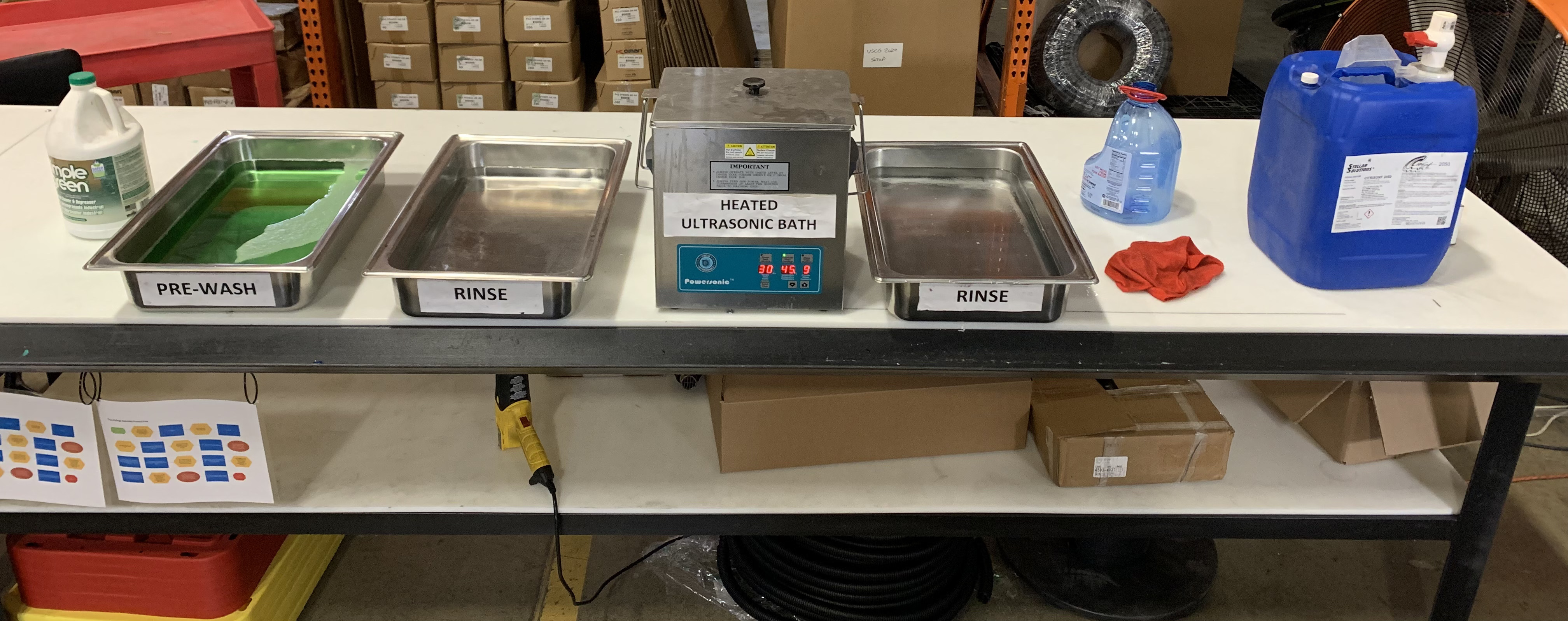
The passivation process typically involves three main steps: preparation and cleaning, applying the passivation solution, and rinsing and drying. Each step is critical to ensuring the effectiveness of the treatment and the long-term performance of the stainless steel parts.
1) Preparation and Cleaning
Before passivation, it’s essential to thoroughly clean the stainless steel parts to remove any dirt, oils, or grease. This ensures that the passivation solution can work effectively and form a uniform oxide layer.
2) Applying Passivation Chemicals
Stainless steel parts are immersed in a passivation solution, such as citric or nitric acid, depending on the desired outcome. The immersion time and concentration must be carefully controlled to achieve optimal results.
3) Rinsing and Drying
After the passivation process, parts must be thoroughly rinsed to remove any residual chemicals. Proper drying is also important to prevent water spots and ensure the integrity of the passive layer.

The Challenges of Passivation
Despite its benefits, passivation can present some challenges. For example, regular maintenance and reapplication may be necessary, especially in harsh environments. Also, improper passivation can lead to issues like discoloration or reduced corrosion resistance.
Potential Issues and How to Avoid Them
Common problems include incomplete removal of free iron or improper solution concentration. To avoid these, it's important to follow established guidelines, use the right equipment, and work with experienced professionals who understand the process.
Safety Measures During Passivation
Safety is a top priority when handling passivation chemicals. Always wear appropriate personal protective equipment (PPE), such as gloves and goggles. Ensure good ventilation to avoid inhaling fumes. Follow manufacturer instructions for chemical concentrations and disposal procedures to maintain a safe working environment.
Key Equipment in Stainless Steel Passivation
 In industrial passivation setups, specialized equipment is essential. This includes acid-resistant tanks for immersion, spray rinse stations for thorough washing, and drying ovens to eliminate moisture. Automated systems like conveyors and robotic arms help streamline the process, while pH meters and solution analyzers ensure consistent quality.
In industrial passivation setups, specialized equipment is essential. This includes acid-resistant tanks for immersion, spray rinse stations for thorough washing, and drying ovens to eliminate moisture. Automated systems like conveyors and robotic arms help streamline the process, while pH meters and solution analyzers ensure consistent quality.
Frequently Asked Questions
What is passivation?
Passivation is a chemical process that removes free iron from stainless steel surfaces and forms a protective oxide layer, improving corrosion resistance and extending the material's lifespan.
Does stainless steel need passivation?
Yes, passivation is recommended after fabrication or machining to enhance the performance and durability of stainless steel components.
What chemicals are used in passivation?
Common chemicals include citric acid, nitric acid, and sodium dichromate, each offering different benefits based on the application and environment.
Can I passivate carbon steel?
Carbon steel doesn’t form a natural passive layer like stainless steel, so passivation is typically done through coatings or other treatments, rather than chemical oxidation.
What Factors Influence the Success of the Passivation Process?
Factors include the chromium content of the steel, correct immersion time, surface condition, temperature, and solution strength. Each plays a role in the effectiveness of the passivation treatment.
Glass Jar Blender,Glass Jug Food Blender,Large Glass Blender Jar,Jug Blender Glass Jar
Jiangmen Sanxin Appliances Co.,Ltd , https://www.sanxinfty.com